Cytoskeleton and Cell Motility
V.1 Coordinators
V.2 Participants
V.3 Introduction
V.4 Specific Research Objectives
V.5 Background and Significance
V.6 Research Plan
- V.6.iii Subproject 3 - What Mechanisms Drive the Movement and Positioning of Subcellular Elements?
- V.6.iii.a Fast Axonal Transport
- V.6.iii.b Vesicle Transport in Embryos
- V.6.iii.c Modeling Transport Mechanisms
V.8 Timeline
< Previous | Page 12 of 23 | Next >
V.6.iii.a.2.ii Functional analysis in vivo:
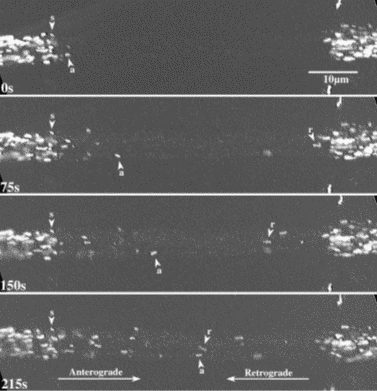
We test the function of each identified component by tracking the motion of specifically labeled organelles in the axons of Drosophila larval nerves. The narrow and extremely elongated structure of axons constrains motion to essentially one dimension. Microtubules in axons are relatively stable and organize with minus ends toward the cell body and plus ends toward the terminal (Heidemann et al ., 1981). This geometry greatly simplifies data collection, analysis, and interpretation, which should in turn simplify models of transport. We have fly strains that express transgenic fluorescent fusion proteins targeted to three different types of organelles (A. Pilling and W. Saxton in preparation; K. Broadie unpublished): mitochondria (CytC Oxidase-GFP), and two different transport vesicles (nSynaptobrevin-GFP and Synaptotagmin-GFP). We control theexpression of those transgenes by a tissue-specific Gal4-UAS promoter system (Brand and Perrimon, 1993). Thus we can focus on transport of the fluorescent organelles in a single cell type (e.g. motor neurons) in complex tissues or mixtures of cells. Time-lapse fluorescence microscopy records organelle behavior at 1 frame/sec (Figure V.6) and we track organelles frame to frame manually (Figure V.7).
Analysis of the tracking data derives several parameters of motion: run directions (anterograde or retrograde), run frequency, run duration, mean and peak velocities, pause frequencies, and frequencies of direction reversal (Pilling in preparation; Gross et al ., 2000). This tracking and analysis system is reliable but would benefit from an increase in time resolution and from automated tracking software. We propose to improve our fluorescence imaging to attain a collection rate of 10 or more frames/sec (Callamaras and Parker, 1999), and to develop software for automated tracking of fluorescent organelles. We will also develop optical trapping approaches to measure forces on GFP-labeled organelles in axons (Welte et al ., 1998) in intact nerves and in primary cultures of differentiated single Drosophila neurons.
| Fig. V.6. A portion of a Drosophila segmental nerve that was actively transporting GFP-filled mitochondria toward microtubule plus ends (anterograde, a) and toward minus ends (retrograde, r). Many mitochondria were stationary (s). To allow better tracking of moving mitochondria, the center part of the nerve was photobleached. |
 |
| Fig. V.7. Tracking plots for individual mitochondria in segmental nerves. |
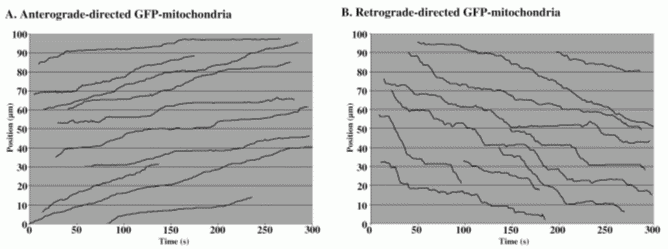 |
The GFP-organelle transport system is ideal for extended analyses of motility mechanisms. It will allow observation of the effects of disruption of many different components of the transport machinery and can be done with multiple specific organelle types, limited only by our ability to find organelle-specific fusion partners for GFP. Our analysis of tracking data to date indicates that cytoplasmic dynein carries mitochondria and both types of vesicle retrograde (toward the cell body). Unc104 carries only synaptotagmin vesicles anterograde (toward the terminal) while conventional kinesin carries mitochondria and both vesicle types anterograde. Kinesin appears to be required for dynein-based transport toward the cell body. We suspect that dynein must be transported away from the cell body before it can actively move back toward the cell body (toward minus ends) with its cargoes. Dynein mutations alter some parameters of anterograde mitochondrial transport, suggesting that dynein occasionally competes with conventional kinesin. Our ability to recognize and interpret these and other subtle changes in transport in mutants depends on compiling large sets of tracking data, which should benefit greatly from more sensitive recording, automated tracking software, and appropriate models.