Cytoskeleton and Cell Motility
V.1 Coordinators
V.2 Participants
V.3 Introduction
V.4 Specific Research Objectives
V.5 Background and Significance
V.6 Research Plan
- V.6.iv Subproject 4 - Experiments on the Actin Cytoskeleton
- V.6.iv.a Introduction
- V.6.iv.b Background and Significance
- V.6.iv.c Aims
- V.6.iv.d Biochemical Assembly of F-actin: Thermodynamics
- V.6.iv.e Construction of a Composite Actin Network to Mimic Cytoskeletal Mechanics
V.8 Timeline
< Previous | Page 15 of 23 | Next >
V.6.iv.d Biochemical Assembly of F-actin: Thermodynamics:
V.6.iv.d.1 Introduction:
To begin to understand the actin cytoskeleton, we must understand actin itself in biochemical and biophysical detail. Self-assembly of globular actin (G-actin) into long and stiff actin filaments (F-actin) is central to the roles that this abundant protein plays in cells (Sheterline, 1994; Stossel, 1984). In vitro assembly of purified actin suggests that actin polymerizes via condensation, much like the gas to liquid phase transition (Oosawa, 1993). In this model, G-actin molecules in solution act like a gas, while actin protomers in F-actin behave as if they were in a liquid or solid condensed state. Thus, actin polymerization would be a first order phase transition, with a certain equilibrium constant at defined ionic conditions. For example, the critical concentration, which is equivalent to the dissociation constant of G-actin from F-actin, is a fraction of a micromolar in a typical solution condition of 100 mM KCl, 2 mM MgCl2, 0.2 mM CaCl2, 0.2 mM ATP, and Tris-Cl at pH=8 (Pollard, 1986; Carlier, 1994). Oosawa and colleagues demonstrated that they could separately determine the entropy and enthalpy changes associated with actin polymerization by applying van't Hoff's law, following measurements of the critical concentration in the temperature range over which the protein remains stable (Oosawa, 1975). The enthalpy of actin polymerization is approximately 20 kcal/mole, and the entropy change is about 0.1 kcal/mole/K, so actin polymerization is endothermal, i.e., the solution absorbs heat from the environment; but with a large entropy gain, i.e., more random states are available in a solution of F-actin than of G-actin. This counterintuitive picture holds because as G-actin polymerizes it releases a large number of bound water molecules.
However, we need to reexamine these thermodynamic predictions for actin polymerization because the original experiments and analysis preceded the discovery of the coupling of ATP hydrolysis to actin polymerization (Wegner, 1982). When G-actin with one ATP molecule bound in its central cavity attaches to an existing filament, ATP hydrolyzes simultaneously, and a slow release of the phosphate group (pi) follows (Pollard, 1986; Carlier, 1986). Therefore, the simple estimate of both enthalpic and entropic changes due to actin polymerization from critical concentration measurements of ATP actin is incorrect. A correct measurement is essential to understand the coupling of ATP hydrolysis to dynamic actin assembly and eventual force generation at the cell leading edge.
The coupling between actin polymerization and ATP hydrolysis is also important for defining the mechanical properties of F-actin and the actin network. About half of the total actin in the cytoplasm is in filaments. Most of the pre-existing F-actin is in ADP form, which is mechanically and biochemically less stable, whereas newly polymerized filaments are either ATP actin, or ADP-Pi actin since the release of Pi is relatively slow. The mechanical strength of F-actin seems to differ in ATP as opposed to ADP solution (Gershman, 1989; Janmey, 1990). This complex behavior couples with a large array of small auxiliary actin binding proteins, such as profilin (Kinosian, 2000; Vinson, 1998), thymosin-β (Pantaloni, 1993; Carlier, 1993), and actophorin/cofilin/ADF (Maciver, 1994), which regulate cellular actin dynamics by their differential affinity to ADP vs. ATP actin.
V.6.iv.d.2 Preliminary Results:
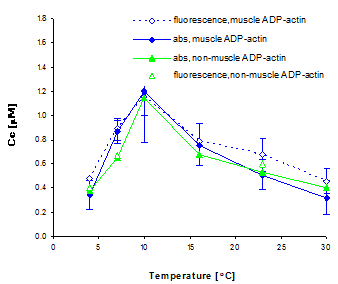
The relationship between the critical concentration of ADP actin and temperature is surprisingly non-monotonic (Figure V.9):
|
Fig. V.9. Critical concentration of ADP actin as a function of temperature. We induced polymerization of ADP bound G-actin by adding 50 mM KCl, and measured the critical concentration by both absorbance and fluorescence assays. The absorbance assay combined ultra-centrifugation and the Bio-Rad protein detection assay. The fluorescence assay was performed on 20% pyrine labeled actin. We have confirmed the temperature dependence of the critical concentration on several actin preparations. We also observed similar behavior for ATP bound actin, which contradicts results from earlier biochemical studies using less direct methods. In light of this new finding, we propose that actin undergoes a conformational change at around 10-15°C. |