Cytoskeleton and Cell Motility
V.1 Coordinators
V.2 Participants
V.3 Introduction
V.4 Specific Research Objectives
V.5 Background and Significance
V.6 Research Plan
- V.6.iv Subproject 4 - Experiments on the Actin Cytoskeleton
- V.6.iv.a Introduction
- V.6.iv.b Background and Significance
- V.6.iv.c Aims
- V.6.iv.d Biochemical Assembly of F-actin: Thermodynamics
- V.6.iv.e Construction of a Composite Actin Network to Mimic Cytoskeletal Mechanics
V.8 Timeline
< Previous | Page 17 of 23 | Next >
V.6.iv.e Construction of a Composite Actin Network to Mimic Cytoskeletal Mechanics:
V.6.iv.e.1 Introduction:
The unique network of actin filaments (F-actin) and actin-binding proteins is a major cytoskeletal component that defines the mechanical properties of cell (Alberts, 1994). At the leading edge in many eukaryotic cells, a dynamic network primarily of actin and actin binding proteins induces motility (Condeelis, 1993; Small, 1989; 1995). Characterizing the mechanical and rheological properties of the actin network as cross-linked by various actin-binding proteins, is therefore an important step towards understanding cell mechanics.
Filamin and alpha-actinin represent two distinct classes of actin cross-linking proteins which affect the mechanical properties of cells (Stossel, 2001; Lebart, 1993). The 270 kDa large actin cross-linking protein filamin forms a dimer, which then cross-links two actin filaments at wide and variable angles, much like a molecular coil spring (Gorlin, 1990). An appropriately constituted F-actin/filamin network has the largest elastic modulus among actin gels. In a human melanoma cell line deficient in filamin, cells suffer constant uncontrollable protrusions, a process known as blebbing (Cunningham, 1995). Alpha-actinin, on the other hand, represents a class of actin cross-linking proteins, which often induce formation of loose actin bundles, including stress fibers (Blanchard, 1989; Pavalko, 1991). These bundles are often dynamic in size and structure, affecting cell properties like polarity and overall shape.
V.6.iv.e.2 Preliminary Results:
Preliminary experiments have revealed the amazing resilience of an actin/filamin network. Under appropriate conditions, a mixture of actin/gelsolin/filamin can form a crosslinked network that withstands repeated deformations of over 100% strain (Figure V.10). No other actin crosslinking proteins including alpha-actinin, arp2/3 complex, and streptavidin using partially biotinylated actin behave similarly.
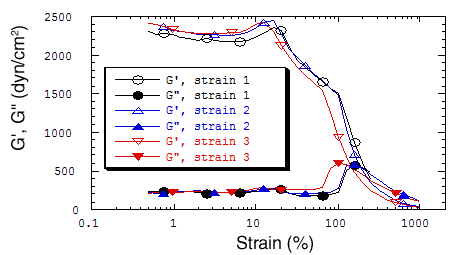
|
| Fig. V.10. Resilience of a filamin/actin network. A mixture containing purified actin, filamin, and recombinant gelsolin was polymerized on an RSF-II rheometer plate for 2 hours prior to three consecutive strain sweeps. The molar ratio of the three proteins was approximately 1:1.3:500 for filamin:gelsolin:actin with an actin concentration of 1 mg/ml. No other crosslinked actin network has been found to withstand over 100% strain. |
V.6.iv.e.3 Hypothesis:
Our primary hypothesis concerning the mechanism of the extraordinary elasticity of the filamin-actin gel is that the two floppy arms of the filamin dimer enable the actin filaments to remain crosslinked even when the filaments rotate greatly with respect to each other. The known structure of nonmuscle human filamin (which the older literature refers to as ABP-280 to distinguish it from muscle filamin, which lacks a 24 amino acid hinge domain) strongly supports this hypothesis. We will explore the factors that can reduce the network strength by diminishing the crosslinking activity of non-muscle filamin. Phosphorylation of filamin reduces its affinity for F-actin, but its effect on crosslinking remains untested. The exposed hinge 1 of non-muscle filamin between repeats 15 and 16 contains a calpain cleavage site. We expect the effect of cleavage on rheological properties to partially reveal the structural basis of the unusually strong and resilient gelation activity of intact non-muscle filamin.