Cytoskeleton and Cell Motility
V.1 Coordinators
V.2 Participants
V.3 Introduction
V.4 Specific Research Objectives
V.5 Background and Significance
V.6 Research Plan V.7 Relation with Organogenesis (project 3) and Biological Networks (project 1)
V.8 Timeline
< Previous | Page 21 of 23 | Next >
V.6.vi Subproject 6 - Intracellular Study of Cytoskeletal Viscoelastic Properties:
V.6.vi.a Overview:The Forgacs group has recently designed and constructed a powerful translational magnetic tweezers that allows the experimental biophysical study of the cytoskeleton inside living cells (see Figure V.12; Hosu et al., 2002).
A magnetic tweezers is a device capable of exerting a force of controlled magnitude and direction on magnetic particles. Due to the simplicity of their operation, low cost of construction and versatility magnetic tweezers have recently gained popularity in probing biological materials at various scales. Most applications to date address the physical properties of (i) single molecules, (ii) filamentous macromolecular networks or (iii) cell surfaces. Very few tweezers explore the intracellular environment (Bausch et al. 1999; Moller et al., 2000; Kuznetzov and Hasenstein, 2001). Other powerful techniques, like optical tweezers and atomic force microscopes can also be used for applications (i-iii). The advantage of magnetic micromanipulators becomes evident in investigating intracellular properties. Optical tweezers exert force on microscopic objects that are transparent to (laser) light. Since many such objects reside inside a cell, optical tweezers cannot selectively operate in the intracellular environment. AFMs cannot probe the interior of a cell.
|
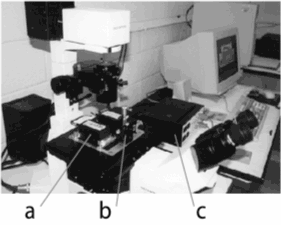
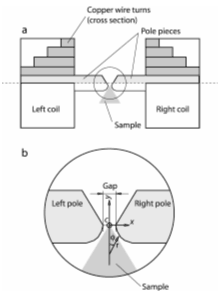
| Fig. V.12 Left panel. Magnetic tweezers work station: Measuring unit, composed of two coils (a) and a sample holder (b); c-control unit. Right panel. Measuring unit: (a) Top view; (b) Pole pieces detail; c - Central point. |
A typical intracellular application delivers magnetic beads by electrophoresis, phagocytosis or injection into living cells and translates or rotates them with the tweezers. We record the trajectory of motion and analyze it in terms of well-established models of viscoelasticity (Fung, 1993). This approach yields local information on the viscosity and elastic properties of the cytoplasm. We can assess the contribution of various intracellular components (e.g. cytoskeletal filaments like actin or microtubules) by controlled modifications of the cell's interior (e.g. use of cytoskeleton depolymerizing agents or by functionalizing the beads to bind to specific molecules). Beads of varying size can provide information on the local intracellular geometry, typically determined by the cell's cytoskeleton. Systematic measurements of local viscoelastic parameters can reveal abnormal transformations inside the cell.
Magnetic micromanipulators can either rotate or translate particles. Rotation reveals the viscoelastic properties of the bead's environment. To displace a magnetic particle is more complicated, requiring a spatially varying magnetic field. The recorded trajectory of the particle then determines the viscoelastic properties.
Our tweezers have several advantages: (i) They use two coils (as opposed to four in other devices) which simultaneously magnetize the particles and due to the special shape of the pole pieces (see Figure V.12) provide a constant magnetic gradient (needed to exert translational force) over cellular dimensions close to the central point along the y-axis (Figure V.12). (ii) The design is versatile and mounts on the stage of an inverted microscope. (iii) It can exert over 600 pN force on a 1 micron diameter particle, an order of magnitude more than similar devices.
V.6.vi.b Preliminary Results and Proposed Measurements:We have used our tweezers to study cytoskeletal viscoelastic properties of macrophages and CHO (Chinese Hamster Ovary) cells. Both cell types readily phagocytose micron size beads. Figure V.13 shows the intracellular displacement of beads and their recorded trajectory.
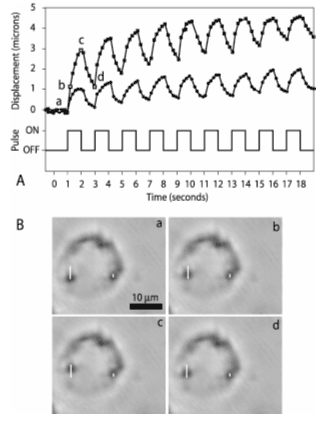
| Fig. V.13 Tweezers induced displacement of beads inside a macrophage. A. Intracellular trajectory of two superparamagnetic beads (top traces) during magnetic pulses (bottom trace). B. Sequence of images showing bead displacements. The two white vertical lines denote the total displacement of each bead; a, b, c and d are marked by open squares and open circles on the top traces in A, for the left and the right bead, respectively. Imaging used bright field microscopy with a 60X objective. Bright field microscopy provides fewer details inside the cell than phase contrast, facilitating bead tracing. 3A, 1 Hz current pulses with a 200 µm gap distance between poles (see Figure V.14 produce a 220 pN force on 1.28 µm beads. The cells were located 300 µm from the central point of the tweezers along the midline between the poles (see Figure V.14). |
The displacement curves in Fig. V.13 are characteristic of viscoelastic materials. We will perform similar measurements with the embryonic cells studied in Project 3 (Organogenesis) to provide quantitative input for modeling. These experiments will complement the ones that utilize the parallel plate compression apparatus to yield tissue level viscoelastic characteristics (see Project 3). Models of organogenesis must provide experimentally testable predictions. One such prediction might be the effect on limb development of cytoskeletal changes in the pre-cartilage limb bud mesenchyme due to actin depolymerizing agents like latrunculin. Magnetic tweezers experiments will provide the quantitative information for modeling latrunculin triggered cytoskeletal changes.
The quantitative study of cross-linked actin networks, in Subproject 4 is performed outside the cell. We will to compare those results with intracellular data to assess the relevance of extracellular studies.