Organogensis
VI.1 COORDINATORS
VI.2 PARTICIPANTS
VI.3 SUMMARY
VI.4 INTRODUCTION
VI.5 SPECIFIC AIMS
VI.6 BACKGROUND AND SIGNIFICANCE
- VI.6.i General Background to Organogenesis
- VI.6.ii Interplay of Physical and Genetic Mechanisms in Organogenesis
- VI.6.ii.a Cell Adhesion
- VI.6.ii.b Chemotaxis and Haptotaxis
- VI.6.ii.c Epithelial and Mesenchymal Tissues
- VI.6.ii.d Epithelia and Cell Polarity
- VI.6.ii.e Mesenchyme and Extracellular Matrix
- VI.6.ii.f Mesenchymal Condensation
- VI.6.ii.g Cell Excitability
- VI.6.ii.h Reaction-Diffusion Mechanisms
- VI.6.ii.i Differentiation
- VI.6.ii.j Summary of Basic Mechanisms of Pattern Formation and Morphogenesis
- VI.6.ii.k Neuronal Guidance, Fasciculation and Defasciculation
- VI.6.iii Background to Specific Experimental Systems
VI.8 PRELIMINARY RESULTS
VI.9 RESEARCH DESIGN AND METHODS
VI.10 RELATIONSHIP TO CYTOSKELETON (PROJECT 2) AND BIOLOGICAL NETWORKS (PROJECT 1)
VI.11 TIMELINE
< Previous | Page 6 of 27 | Next >
VI.6.iii.b Limb Development:
Intensive research on the development of the vertebrate limb skeleton, has identified many factors that affect its symmetries and polarities (reviewed in Ng et al., 1999). However, we still do not understand the dynamical process by which the interactions of limb bud cells with various factors they produce result in a series of articulated, well-arranged rods and nodules of cartilage. Limb bud mesenchyme exhibits self-organization, so the cell surface-extracellular matrix (ECM) adhesive interactions that promote precartilage condensation in vivo also occur in a patterned fashion in isolated mesenchyme in vitro.
Precartilage mesenchymal cells first form tight aggregates or condensations (Hall and Miyake, 1992; 2000; Newman and Tomasek, 1996), then differentiate into cartilage, establishing the primordia for the bony skeleton. Chondrogenesis in vivo requires transient condensation (Hall and Miyake, 1992; 2000; Newman and Tomasek, 1996), and also in vitro, unless circumvented experimentally (Zanetti and Solursh, 1984). Precartilage condensation reflects changed cell-ECM and cell-cell interactions (Frenz et al., 1989a, b; Mackie et al., 1987; Oberlender and Tuan, 1994; Widelitz et al., 1993), and leads to cytoskeletal-linked changes in cell shape (Zanetti and Solursh, 1984). Once condensations have formed, a cascade of signals is entrained that ultimately results in the differentiation of the mesenchymal foci into cartilage (Smales and Biddulph, 1985; Gay and Kosher, 1985; Leonard and Newman, 1987; Zhang et al., 1996).
|
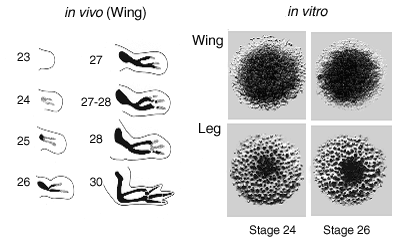
| Fig VI.2 Left, Progress of chondrogenesis in the chick wing bud between days 4 and 7 of development. Solid black regions represent definitive cartilage; stippled areas represent precartilage condensation. Stages are those of Hamburger and Hamilton. Right, Morphology of representative Alcian blue-stained micromass cultures, prepared from mesenchyme isolated from the distal tips of stages 24 and 26 wing and leg buds and grown for six days in serum-free medium. Macroscopic images; culture diameters approximately 5 mm (Downie and Newman, 1994). |
Chicken precartilage mesenchyme in culture forms a continuous sheet or a set of isolated nodules of cartilage, depending upon whether it is derived from the wing or leg bud (Downie and Newman, 1994; 1995) (Fig. 1). What determines where and when foci of condensation initiate?
Earlier work suggested that the ECM protein fibronectin mediates condensation (Frenz et al., 1989a,b; Downie and Newman, 1995; Gehris et al., 1997). These studies, however, were based on interference with endogenous fibronectin; analysis of effects of overexpression of specific fibronectin domains, which we propose, would make a more convincing case. Recent work also strongly suggests that reaction-diffusion organizes limb skeletal patterning (Miura and Shiota, 2000a) in which the activator is one or more molecules of the TGF-β family (Miura and Shiota, 2000b), and the lateral inhibitor, whose molecular identity is currently unknown, is elicited by FGFs interacting with FGF receptor 2 at condensation sites (Moftah et al., 2002). These findings provide a basis for modeling limb skeletal pattern formation using the CPM framework, in conjunction with information on the cohesivity and viscoelastic properties of limb bud precartilage mesenchyme. Measurement of these parameters is also described in our plans, below.