Organogensis
VI.1 COORDINATORS
VI.2 PARTICIPANTS
VI.3 SUMMARY
VI.4 INTRODUCTION
VI.5 SPECIFIC AIMS
VI.6 BACKGROUND AND SIGNIFICANCE
- VI.6.i General Background to Organogenesis
- VI.6.ii Interplay of Physical and Genetic Mechanisms in Organogenesis
- VI.6.ii.a Cell Adhesion
- VI.6.ii.b Chemotaxis and Haptotaxis
- VI.6.ii.c Epithelial and Mesenchymal Tissues
- VI.6.ii.d Epithelia and Cell Polarity
- VI.6.ii.e Mesenchyme and Extracellular Matrix
- VI.6.ii.f Mesenchymal Condensation
- VI.6.ii.g Cell Excitability
- VI.6.ii.h Reaction-Diffusion Mechanisms
- VI.6.ii.i Differentiation
- VI.6.ii.j Summary of Basic Mechanisms of Pattern Formation and Morphogenesis
- VI.6.ii.k Neuronal Guidance, Fasciculation and Defasciculation
- VI.6.iii Background to Specific Experimental Systems
VI.8 PRELIMINARY RESULTS
VI.9 RESEARCH DESIGN AND METHODS
VI.10 RELATIONSHIP TO CYTOSKELETON (PROJECT 2) AND BIOLOGICAL NETWORKS (PROJECT 1)
VI.11 TIMELINE
< Previous | Page 8 of 27 | Next >
VI.6.iii.d Cardiovascular Development:
The cardiovascular system, comprising the heart and its associated network of blood vessels, forms the vertebrate embryo's first functional organ. Over the last several centuries, its embryonic development has sparked the interest and imagination of many great minds, including Leonardo da Vinci. While observation and embryonic manipulation have revealed much, only the advent of molecular biology has made the study of the genetic pathways involved possible.
VI.6.iii.d.1 Early vertebrate heart development:Over the last century, embryologists documented the morphology of the developing amphibian heart. Subsequent studies revealed that the morphogenesis of the heart is remarkably similar across species. Likewise, the gene families currently identified as being involved in cardiogenesis are highly conserved. We choose Xenopus as an example.
Cell explantation and lineage tracing experiments show that the cells destined to contribute to the developing heart originate in two bilateral regions within the dorsal mesoderm located initially on either side of the Spemann organizer (Fig. VI.6.3A). The cells in these "presumptive heart fields" of lateral plate mesoderm are specified during gastrulation (stage 10 in Xenopus development) and are competent to form beating heart tissue even if excised from the embryo (Goerttler, 1928; Bacon, 1945; Jacobson, 1960; Jacobson and Sater, 1988). However, differentiation occurs much later, around stage 27 (Figure VI.3C), after the cardiogenic cells migrate to the ventral midline (Figure VI.3 B). At this time, the cells fated to contribute to the muscular layer of the heart, the myocardium, begin to express markers of terminal myocardial differentiation, such as cardiac troponin I (TnIc), myosin heavy chain alpha (MHCα and myosin light chain 2 (MLC-2) (Drysdale et al., 1994; Logan and Mohun, 1993; O'Brien et al., 1993; Chambers et al., 1994). Simultaneously, the differentiating fields continue to move toward the ventral midline, delaminate from the surrounding tissues, and form an epithelial layer (Davenport, 1895). These two differentiating patches of cells fuse into a single cardiogenic plate, which then rolls to create a lumen - the primitive heart tube. Rhythmic beating of the primitive heart tube occurs in Xenopus at stage 32 and morphogenetic movements cause the heart tube to loop, further defining the chambers and resulting in a fully functional embryonic heart by stage 35 (Figure VI.3 E). Thus, the early development of the vertebrate heart relies heavily upon physical mechanisms, including cell migration, separation (delamination) of tissues, and lumen formation.
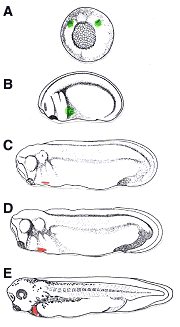
|
Fig VI.3 Heart development in Xenopus laevis (A) Specification of the heart primordia (marked in green) occurs at gastrulation in the two regions of the mesoderm directly adjacent from the dorsal lip (St. 10.5 Xenopus gastrula drawn) (B) These presumptive heart fields (green) migrate ventrally during neurulation. (C) Myocardial differentiation markers (red) are first expressed at around stage 27 of Xenopus development. (D) Fusion of the differentiating heart fields occurs between stages 28 and 29 of development. (E) By stage 35 of Xenopus development, the primitive heart tube as begun rhythmic contractions and has undergone looping to form a functional embryonic heart. (Drawings adapted from Nieuwkoop, P.D., and Faber, J. 1994. Normal table of Xenopus laevis (Daudin), 2nd ed. Garland Publishing, Inc., New York.) |
VI.6.iii.d.2 Trabeculation:
Ventricular trabeculation-compaction is an important morphogenetic process at midgestation (Srivastava and Olson, 2000; Icardo, 1984; Berne, 1979; Rumyantsev, 1991). At the final stages of cardiac looping (E9.5), the myocardium is still a compact epithelial tissue layer containing myocytes exclusively. Cardiac jelly fills the space between myocardium and endocardium (Icardo, 1984). Initially, the endocardium penetrates through the cardiac jelly and evaginates at discrete points of myocardium, forming outpockets directed toward the myocardium which separate myocardial cells and further penetrate the myocardial wall, expanding laterally into intercellular spaces. Then the myocardium separates into two zones: a compact outer layer and a spongy or trabeculated inner layer. Later (i.e., E13.5-E15.5 of the mouse embryo), most trabeculae in the developing myocardium gradually condense and compact toward the epicardial surface, reducing the intertrabecular recesses to capillaries. The provisional trabeculae of the embryonic myocardium may transform into papillary muscle and contribute to the muscular portion of the interventricular septum. The formation of trabeculae and their subsequent compaction seem critical to ventricular chamber and wall maturation.
Several growth factors are necessary to trabecular development, including neuregulin (Gassmann et al., 1995; Zhao et al., 1998) and its receptors ErbB (Lee et al., 1995), vascular endothelial growth factor (VEGF) (Ferarra et al., 1996), and angiopoietin-1 (Suri et al., 1996). Mutant mice deficient in these genes have severe defects in ventricular trabeculation. Neuregulin and VEGF are growth factors secreted from the endocardium. Neuregulin or VEGF probably signal the myocardium to start trabeculation.
Taber and Zahalak have modeled the myocardium as a thin viscoelastic membrane consisting of contractile (stress) fibers embedded in an isotropic incompressible matrix, with the interaction of myocardial cells and cardiac jelly fibers providing long-range mechanical effects (Taber and Zahalak, 2001). They assume the stress fibers exert their contractile force on the descending limb of their stress-stretch curve. Mechanical instability due to the effectively negative stiffness then creates the trabeculae.