Organogensis
VI.1 COORDINATORS
VI.2 PARTICIPANTS
VI.3 SUMMARY
VI.4 INTRODUCTION
VI.5 SPECIFIC AIMS
VI.6 BACKGROUND AND SIGNIFICANCE
VI.7 THEORETICAL FRAMEWORK
VI.8 PRELIMINARY RESULTS
VI.9 RESEARCH DESIGN AND METHODS
- VI.9.ii Limb Development
- VI.9.ii.a Overview
- VI.9.ii.b Computational Approach
- VI.9.ii.c Subprojects
- VI.9.ii.c.1 Subproject 1 — Role of fibronectin in precartilage condensations
- VI.9.ii.c.2 Subproject 2 — Activator-inhibitor interactions in skeletal pattern formation
- VI.9.ii.c.3 Subproject 3 —Viscoelasticity of limb bud tissues
- VI.9.ii.c.4 Subproject 4 — The Genetic Control of Limb Development
- VI.9.ii.c.5 Subproject 5 — Complete model for avian chondrogenesis
- [ Complete VI.9 Outline ]
VI.11 TIMELINE
< Previous | Page 19 of 27 | Next >
VI.9.ii.c.3 Subproject 3 -Viscoelasticity of limb bud tissues:
To determine the adhesion, dissipative and elastic constants in the CPM we must measure cellular and tissue viscoelastic properties. The Forgacs laboratory has developed several techniques to do so reliably.
We have measured the liquid and viscoelastic properties of dissociated embryonic chicken tissues using the compression plate apparatus schematically illustrated in Fig. VI.7 (Foty et al., 1994, 1996; Forgacs et al., 1998). We are presently applying the same method to intact embryonic chicken limb bud tissue. (Project 2, Section V.6.vi describes measurements of cellular viscoelastic properties using magnetic tweezers).
|
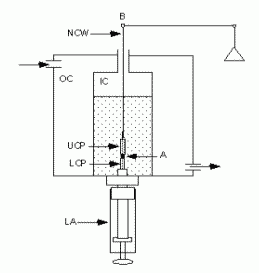
Fig.VI.7. Spherical aggregates are compressed between the parallel plates in the inner chamber (IC). A circulating water bath feeds the outer chamber (OC) with 37°C water. Coating the compression plates with poly(2-hydroxyethylmethacrylate) [poly(HEMA)] (Folkman and Moscona, 1978) minimizes their adherence to the cell aggregates. The upper compression plate (UCP) hangs from the arm (B) of an electrobalance (through a nickel-chromium wire, NCW). Recording its apparent weight establishes a precompression zero force baseline. Raising the lower compression plate (LCP) by turning the lower assembly (LA) compresses the aggregate between the two plates. Two video cameras at 90° angles, one of with a horizontal microscope, monitor the shape of the aggregate. A computer continuously (every 0.6 s) records changes in the force exerted by the aggregate upon the UCP, equal to the changes in the latter's apparent weight. |
Intact tissue fragments from early limb bud and flank of chick embryos (pre-cartilage limb bud mesenchyme) placed in a shaker bath round up overnight into spheres as predicted by Steinberg's Differential Adhesion Hypothesis (DAH). Following compression of these spherical tissue fragments the initial magnitude of the compressive force relaxes to equilibrium in a manner typical of viscoelastic materials (Forgacs et al., 1998). The equilibrium value of the compressive force and the shape of the compressed tissue determine the surface tension (the interfacial tension between the tissue and the surrounding tissue culture medium) through Laplace's equation (Rowlinson and Widom, 1989).
This information suffices to determine the cohesive characteristics of the various tissues involved in limb development (i.e. pre-cartilage limb bud mesenchyme and flank). The cellular interaction parameters, Jτ,τ′, in the CPM relate to the interfacial tensions between the various tissues, whose experimental determination is considerably more complicated. For this task we will prepare similar sized spheroids of tissues that are naturally contact each other during limb development. We will place the spheroids contiguously in the compression apparatus and compress them simultaneously. Following the decay of the compressive force, its equilibrium value provides the interfacial tension between the tissues, which we can use to estimate the binding strength between unit surfaces of adhering cells, a quantity related to Jτ,τ′. To supplement the experiments on mesenchymal condensation described in Section VI.8.ii.c.1 we will perform surface tension measurements with cells deficient in heparan sulfate and expressing various mutated forms of fibronectin.
The specific shape of the compressive force relaxation curve contains information on the viscoelastic properties of the tissue. The corresponding physical parameters explicitly appear in Hdiss as introduced in Eq. (VI.6).