Organogensis
VI.1 COORDINATORS
VI.2 PARTICIPANTS
VI.3 SUMMARY
VI.4 INTRODUCTION
VI.5 SPECIFIC AIMS
VI.6 BACKGROUND AND SIGNIFICANCE
VI.7 THEORETICAL FRAMEWORK
VI.8 PRELIMINARY RESULTS
VI.9 RESEARCH DESIGN AND METHODS
- VI.9.ii Limb Development
- VI.9.ii.a Overview
- VI.9.ii.b Computational Approach
- VI.9.ii.c Subprojects
- VI.9.ii.c.1 Subproject 1 — Role of fibronectin in precartilage condensations
- VI.9.ii.c.2 Subproject 2 — Activator-inhibitor interactions in skeletal pattern formation
- VI.9.ii.c.3 Subproject 3 —Viscoelasticity of limb bud tissues
- VI.9.ii.c.4 Subproject 4 — The Genetic Control of Limb Development
- VI.9.ii.c.5 Subproject 5 — Complete model for avian chondrogenesis
- [ Complete VI.9 Outline ]
VI.11 TIMELINE
< Previous | Page 20 of 27 | Next >
VI.9.ii.c.4 Subproject 4 - The Genetic Control of Limb Development:
VI.9.ii.c.4.i Introduction:
Regeneration and its failure (scarring) provides a window on the patterns of gene expression necessary for limb development by allowing differential studies of attempted regeneration in regeneration competent and incompetent animals.
VI.9.ii.c.4.ii Goal:Identify the genes encoding signaling molecules that operate during regeneration and whose failure causes scarring.
VI.9.ii.c.4.iii Animal and Tissue Models:Currently, we do not know enough about the molecular inventory of genes, proteins, and regulatory pathways stimulatory or inhibitory to regeneration to achieve this goal. An efficient way to gain this knowledge is to study animal models that are strong regenerators. In most vertebrates, regeneration is restricted to a limited number of tissues subject to continual turnover (blood and lymphoid cells, epithelium) or sporadic injury (bone, muscle) (Stocum, 2001). The injury response of most other tissues is scarring. Larval and adult urodeles (salamanders and newts) and anuran tadpoles (frogs and toads), however, are exceptions to this rule. These amphibians can regenerate the spinal cord, neural retina, lens, cardiac muscle, intestine, tail, jaws, and limbs by histolysis of the injured tissues and dedifferentiation of the liberated mature cells to become proliferating mesenchyme-like stem cells (Brockes, 1997; Geraudie and Ferretti, 1998; Stocum, 2001; Chernoff, 1996; Benraiss et al., 1999). These stem cells may be unipotent, redifferentiating only into the cell type of origin, or multipotent, redifferentiating not only into the cell type of origin, but also transdifferentiating into cell types other than the one of origin.
Mammals have latent regenerative capacity that is suppressed (Gage, 2000). In the limb, the gene Msx1 is active only in undifferentiated mesenchyme of the early limb bud of all vertebrates and the limb regeneration blastema of amphibians (Robert et al., 1991; Simon et al., 1995). Forced expression of this gene in cultured mouse multinucleated muscle cells results in their cleavage and dedifferentiation into mononucleate myoblasts (Odelberg et al., 2001). Furthermore, extract from regenerating newt limbs primes mouse myotubes to cleave, dedifferentiate, and proliferate (McGann et al., 2001). These observations argue strongly for an evolutionary conservation in mammals of a major part of the unique regenerative mechanisms deployed by amphibians and thus the generality of amphibian models to identify molecules that are up or down regulated to stimulate or inhibit regeneration.
Our research focuses on two amphibians: the frog, Xenopus laevis, and the axolotl, Ambystoma mexicanum. Xenopus is of particular value because many vital tissues regenerate strongly during tadpole stages, but lose this capacity as the animal undergoes metamorphosis. This difference allows a molecular comparison of the same tissues at regeneration-competent vs. incompetent stages. In addition, Xenopus has a large bioinformatics database. The axolotl regenerates throughout its lifetime and thus enables us to carry out similar molecular comparisons between mature, regeneration-incompetent Xenopus tissues and mature, regeneration-competent axolotl tissues. The limb is a complex structure of musculoskeletal tissue, skin, vascular, and peripheral nerves and is thus likely to be a rich source of molecules that promote regeneration.
VI.9.ii.c.4.iv Experimental Approach:Phase 1: The expression profiles of potential genes will be examined in regeneration-competent and regeneration-deficient tissues by real-time quantitative PCR, gene arrays, and in situ hybridization.
Genes expressed at two-fold levels above that in the opposite tissue will be considered genes that have the potential to drive regeneration, and vice versa. We have elected to examine a substantial number of genes found by others to be developmentally regulated during embryogenesis of the tissues of interest (see Tasks). We have also identified and sequenced, by reciprocal subtraction of cDNA libraries, ~1700 clones of genes expressed at regeneration-competent or regeneration-incompetent early stages of hind limb tissues of Xenopus laevis (Nguyen et al., 2001). Fifteen percent of these are redundant sequences. Of the remaining non-redundant sequences, nearly 80% are novel genes. We have gene-arrayed over 6000 clones from the cDNA library generated by subtraction of regeneration-incompetent cDNA from regeneration-competent cDNA and shown that 25% of these are upregulated by a factor of two or more, while the level of expression of other genes remains the same.
Phase 2: genes identified as regeneration-promoting or regeneration-inhibiting will be subjected to gain or loss of function assays.
These assays will be carried out by introducing sense or antisense genes driven by constitutive or tissue-specific promoters into cells in vivo or in vitro. Available methods for gene delivery include lipofection, electroporation, and the construction of transgenic animals. The first two approaches already are in use in the CRBM. Transgenic mouse technology is established and the generation of transgenic amphibia is being developed.
Lipofection of DNA carried in protein expression plasmids is a proven technique for transfecting genes into Xenopus cells. Lipofection is accomplished by injection into cells of a lipid/DNA solution (Brown, 1998). Alternatively, gene constructs can be electroporated into cells or tissues from regeneration-competent and incompetent stages maintained in vitro. A number of Xenopus cultured cell lines exist (Smith and Tata, 1991). Other cell lines can be obtained, and tissue explants can be easily cultured. The transfected cells or tissue explants can then be implanted into regeneration-incompetent tissues, which will be injured or amputated to determine whether the transfected cells can dedifferentiate to become stem cells that transdifferentiate into other cell types.
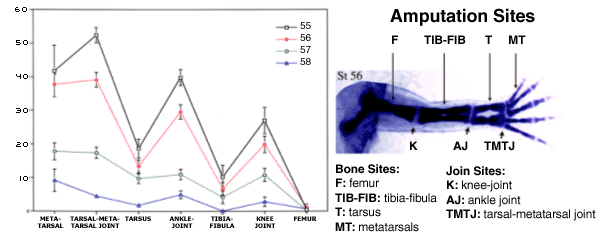
|
| Fig.VI.8a. Xenopus laevis Regeneration Baseline. The graph on the left side of the figure displays the regeneration quality curves for seven amputation sites along the proximo-distal axis of four increasingly older ages of tadpoles: stages 55 through 58. The locations of those amputation sites are shown on the right side of the figure; the skeletal element image is that of a Victoria Blue stained normally developing stage 56 hind limb. As the graph indicates, bony amputation sites' regeneration quality (the "valleys" in the curves) is significantly lower than that of cartilaginous amputation sites (the "peaks" in the curves) (Wolfe et al., 2000; Nye and Cameron, unpublished). |
Transgenesis allows the introduction of foreign genes regulated by tissue-specific or constitutive promoters into the genome of an egg. Constitutive promoters allow these genes to function later in tissues where they might normally be silenced. We will use the Amaya and Kroll (1996) method of restriction enzyme-mediated integration (REMI) to introduce regeneration-promoting genes under the control of a constitutive promoter into Xenopus sperm. In parallel, controls will receive reporter genes encoding GFP. The transfected sperm nuclei are then transplanted into the eggs. Transgenic eggs will be allowed to develop to regeneration-incompetent stages, whereupon regeneration of tissues will be attempted. If the transfected gene(s) function to stimulate regeneration, regeneration competence should be maintained into normally regeneration-incompetent stages. Conversely, experiments designed to test loss of regenerative capacity can be accomplished by making animals transgenic for genes expressed at high levels at regeneration-incompetent stages. These constitutively active genes would be expected to inhibit regeneration at regeneration-competent stages.
Metric Analysis: Quality of regeneration will be assessed by examination of anatomical and histological structure. A quantitative scoring system for regeneration has been developed that is statistically rigorous, based on the anatomy and number of skeletal elements in the regenerated limb (Nye and Cameron, unpublished, Figure VI.8a). Limb function can be evaluated simply by comparing the movements of the regenerated limbs with those of limbs of control animals.