Organogensis
VI.1 COORDINATORS
VI.2 PARTICIPANTS
VI.3 SUMMARY
VI.4 INTRODUCTION
VI.5 SPECIFIC AIMS
VI.6 BACKGROUND AND SIGNIFICANCE
VI.7 THEORETICAL FRAMEWORK
VI.8 PRELIMINARY RESULTS
VI.9 RESEARCH DESIGN AND METHODS>
- VI.9.ii Limb Development
- VI.9.ii.a Overview
- VI.9.ii.b Computational Approach
- VI.9.ii.c Subprojects
- VI.9.ii.c.1 Subproject 1 — Role of fibronectin in precartilage condensations
- VI.9.ii.c.2 Subproject 2 — Activator-inhibitor interactions in skeletal pattern formation
- VI.9.ii.c.3 Subproject 3 —Viscoelasticity of limb bud tissues
- VI.9.ii.c.4 Subproject 4 — The Genetic Control of Limb Development
- VI.9.ii.c.5 Subproject 5 — Complete model for avian chondrogenesis
- [ Complete VI.9 Outline ]
VI.11 TIMELINE
< Previous | Page 21 of 27 | Next >
VI.9.ii.c.5 Subproject 5 - Complete model for avian chondrogenesis:
VI.9.ii.c.5.i Overview:
Based on the results described above, we aim to integrate the various models that together describe the dynamics of biologically relevant molecules, of the cells, cell differentiation, and cell mitosis and apoptosis. The challenge is to come up with an integrated framework with sufficient generality, flexibility and robustness to efficiently carry out computations using the various models to get a complete view of the limb. Obviously, integration involves both theoretical and computational effort.
VI.9.ii.c.5.ii Background:Under the reaction-diffusion scenario the size of the apical zone should significantly affect the pattern in the limb (Newman and Frisch, 1979; Newman et al., 1988), because the number of standing periods of a heterogeneous distribution of a chemical species formed by a reaction-diffusion mechanism depends both on the scale of the basic pattern and on the space available for the pattern to develop. As the limb grows, most cell division occurs in a narrow apical zone, a band of decreasing width confined to the distal tip of the limb bud. How do the changing dimensions of the limb and the apical zone in particular, change the pattern of cell condensation and the number and shape of cell condensation domains? We hope to understand the conditions under which a humerus bifurcates into a radius and ulna and later segments into metacarpals and digits.
Initially we will take the size of the apical zone from experiments, though later we will explore epigenetic mechanisms for sudden spurts of growth and contraction (Newman and Frisch, 1979).
VI.9.ii.c.5.iii Hypothesis:We hypothesize that the basic structure of the skeletal elements will turn out to be due to bifurcations in the pattern as a result of dynamic fluctuations in the length of the apical zone.
VI.9.ii.c.5.iv Model and preliminary results:To grow a skeletal pattern we will need to include several factors. The apical ectodermal ridge (AER), which sits asymmetrically at the tip of the growing limb bud is necessary for proximo-distal development. The AER releases fibroblast growth factors (FGFs) which control mitosis of mesenchymal cells expressing growth receptor 1 (R1) to create an apical zone of cell growth. We will use computational models to study how variations in the size of the apical zone influence the distribution of FGFs and consequently differentiation.
We have simulated mesenchymal cell condensation and the emergence of a skeletal pattern in simple geometries a rectangular three-dimensional domain with multiple grids for various components, specifically, TGF-β, fibronectin and cells.
Initially cells mitose under the influence of FGFs. FGFs upregulate mitosis of mesenchymal cells. The limb includes an apical zone and an active zone. In the active zone TGF-β acts as a reaction-diffusion activator. A chondrogenic pattern appears that precedes the formation of skeletal elements. Cells sensing the TGF-β signal differentiate into cells capable of producing fibronectin. Fibronectin causes cells to become more adhesive to each other and to fibronectin. This feedback obeys an ODE, which represents the biochemistry of the cell-fibronectin binding mediated by FnNTD, and an ODE representing the cell-cell binding mediated primarily by N-cadherin. Growth and movement of cells employ the CPM (Equations VI.1 to VI.9).
With appropriate choice of parameters in Equation VI.10, the activator (TGF-β) concentration produces vertical stripes, a caricature of bone distribution in chicken limb (Figure VI.8a). The right hand side of the split rectangles shows the pre-pattern formed by the morphogen chemical field (TGF-β or fibronectin); the left side shows the organization of mesenchymal cells into pre-cartilaginous tissue in response to the field. Movies are available at: http://www.nd.edu/~lcls. The simulation reproduces the typical bone pattern of one proximal bonelike condensation followed by two bones, and then three digits.
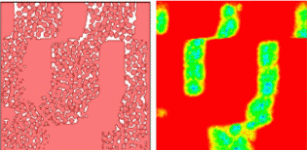
|
Fig.VI.8a Cells respond (left) to FGF field (right), which is secreted by cells in response to TGF-β concentration. Mitosis is turned on, so new cells form and cluster into bonelike patterns. We show only cell cluster boundaries. |
VI.9.ii.c.5.v Plan:
The next aim for the simulation is to allow for three-dimensional computations over more realistic geometrical domains, while maintaining efficiency and scalability. We will extend the model to incorporate AER and FGF fields to allow for the zones of activities to appear as a part of simulations.