Organogensis
VI.1 COORDINATORS
VI.2 PARTICIPANTS
VI.3 SUMMARY
VI.4 INTRODUCTION
VI.5 SPECIFIC AIMS
VI.6 BACKGROUND AND SIGNIFICANCE
VI.7 THEORETICAL FRAMEWORK
VI.8 PRELIMINARY RESULTS
VI.9 RESEARCH DESIGN AND METHODS
- VI.9.iv Cardiovascular Development
- [ Complete VI.9 Outline ]
VI.11 TIMELINE
< Previous | Page 24 of 27 | Next >
VI.9.iv Cardiovascular Development:
VI.9.iv.a Overview:During the midgestation of mammalian embryonic development (E9.5-E15.5), the newly formed embryonic heart is grow rapidly in cell number and size and undergoes a series of morphological and functional changes in response to the increasing volume of circulating blood. The proliferating cardiomyocytes form a distinct loose interwoven meshwork of myocardial fibers, the so-called ventricular trabeculae, in E9.5 ventricular chambers. By E14.5, these trabecular structures become more compact toward the epicardial surface, and the intertrabecular recesses regress to capillaries. The significant reduction of trabeculation in many transgenic and knockout mice is closely associated with the myocardium growth arrest that leads to thinned myocardium and early embryonic lethality between E9.5 and E15.5. On the other hand, the abnormal enhancement of growth activity of myocardium leads to the failure of myocardial compaction, which is likely the cause of a severe pediatric cardiac disease, Noncompaction of the Ventricular Myocardium, in humans. However, we know little about the underlying molecular and cellular mechanisms that regulates myocardial trabeculation and compaction.
VI.9.iv.b Preliminary Results:We engineered mutant FKBP12-deficient mice that have a profound enhancement in ventricular trabeculation (Figure VI.10) and a significant increase in the proliferative activity of cardiomyocytes deficient in FKBP12 (FK506 binding protein-12). A cDNA differential display to identify genes that contribute to the enhanced cardiac growth and ventricular trabeculation in the FKBP12-deficient heart, showed that a TGF-β superfamily member, BMP-10, may be a novel peptide growth factor in cardiac development. BMP-10 is exclusively and transiently expressed in the cardiomyocytes located in the ventricular trabecular myocardium from E9.5 to E15.5, a critical time span when cardiac development shifts its main course from cardiac pattern formation to cardiac growth and chamber maturation. In FKBP12-deficient hearts, BMP-10 expression is prolonged and upregulated.
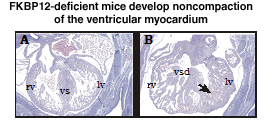 Fig VI.10 Histology of FKBP12-deficient E14.5 embryonic heart. Embryos were fixed in 10% neutral buffered formalin, paraffin embedded and sectioned, and stained with eosin and haematoxylin. A, FKBP12 hetrozygous heart has normal cardiac morphology and histology. B, FKBP12-deficient heart has the noncompaction of the ventricular myocardium with characteristically increased amount and thickness of ventricular trabeculae, which is accompanied by the ventricular spetal defect. vs: ventricular septum; vsd: ventricular septal defect; lv: left ventricle; rv: right ventricle; arrow: abnormal ventricular trabeculae. |
VI.9.iv.c Hypothesis:
Based on our data, we hypothesize that BMP-10 and its associated signaling pathway are critical to the cardiac growth and ventricular trabeculation-compaction in cardiac development at midgestation.