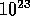 ) molecules.
Thus a statistical approach works very well!
) molecules.
Thus a statistical approach works very well!
Example: Thermodynamic properties of gases
It is totally impractical and not useful to know the exact microstate (positions and momenta of all molecules) of a large volume of gas.
Useful properties are statistical: average energy of particles (temperature), average momentum change from collisions with walls of container (pressure), etc.
The law of large numbers implies the error in the averages decreases
as the number of particles increases.
Macroscopic volume (state) of gas has O(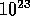 ) molecules.
Thus a statistical approach works very well!
) molecules.
Thus a statistical approach works very well!
A given state or configuration of the system occurs with a particular probability, subject to a probability distribution.